Abstract
In view of the exciting results reported in patients with CD19+ malignancies given CAR T cells, it is expected that a continuously growing number of patients will be offered this treatment and, thus, will be exposed to gene-modified products. Since the techniques of gene manipulation are relatively new, some of the risks associated to CAR T therapy may be unpredictable.
Recently, two patients who relapsed with CD19-, CAR-expressing leukemia were reported, this observation being interpretable in light of an inadvertent leukemic cell transduction with the second generation CAR.CD19 lentivirus during CAR T cell manufacturing (Lacey, ASH, 2016 128:281). Immunoglobulin heavy chain sequencing analysis of 17 additional infusion products also identified the leukemic clonotypes in six additional products (86%). In vitro and in vivo experiments proved that these CAR+ leukemic clones were not killed by CAR.CD19 T cells (Ruella, ASH, 2017 130:4463).
Since lentiviruses proved to be superior for transduction of quiescent hematopietic stem cells due to their ability to infect non-dividing cells, we hypothesized that CAR-T cell manufacturing based on the genetic modification of T cells by gammaretroviral vector could theoretically represent a safe approach.
Peripheral blood or bone marrow (BM)-derived mononuclear cells of patients with >40% of blasts at diagnosis (CD45dim+/CD34+/CD19+/CD22+/CD10+), were transduced with a retrovirus encoding for a second generation CAR.CD19.41bb.z in frame with a suicide gene (i.e., inducible caspase 9, iC9) employed in the academic Clinical Trial (NCT03373071) run at the Bambino Gesù Children's Hospital, Rome, Italy. Patient-derived CAR-T cells showed a phenotype not significantly different from that found on CAR-T cells generated by healthy-donors (data not shown). In particular, we demonstrated that both flow-cytofluorimetry and RealTime-quantitative PCR (with a sensitivity up to 10-5) failed to identify leukemic cells in the final CAR-T cell products generated from Bcp-ALL patients.
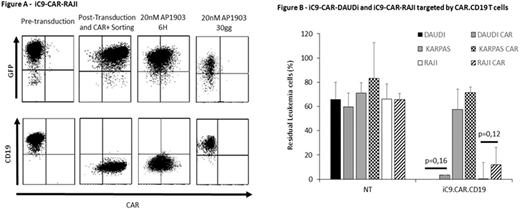
To generate an in vitro model of CAR+ leukemic cells, we genetically modified CD19+ RAJI and DAUDI cell lines with the bicistronic retroviral vector carrying both second generation CAR.CD19 and the suicide gene iC9 (iC9.CAR-RAJI and iC9.CAR-DAUDI). We demonstrated the possibility of promptlyeliminating CAR+ leukemic cells, through exposure to 20nM of AP1903 of iC9.CAR-DAUDI and iC9.CAR-RAJI cells. Indeed, very early activation (6 hours) of the suicide gene iC9 resulted into a significant reduction in the percentage of CAR+ RAJI leukemic cells (Fig.A).
The presence of iC9.CAR.CD19 molecule on leukemic cells precluded the detection of the CD19 antigen, whereas cells retain the expression of all other specific B-lineage markers. CD19 antigen started to be detectable 72 hours after AP1903 exposurewhen CAR negative leukemic cells become preponderant. To demonstrate that CD19 antigen was not down-regulated, but only masked by CAR molecule in iC9.CAR-RAJI and iC9.CAR-DAUDI cell lines, we measured CD19 mRNA, showing no significant modification with respect to wild-type (WT) RAJI and DAUDI cell lines. Moreover, iC9.CAR-RAJI and iC9.CAR-DAUDI cell lines were effectively eliminated by CAR.CD19 T cells (12.5±13.7% and 3.4±4.3% residualleukaemia, respectively) at the same extent of WT cell line (0% and 0.08±0.1%, residual leukaemia, respectively; p>0.05 Fig.B).
To assess if patient-derived iC9.CAR.CD19 T cells were able to generate leukemia in vivo mouse model, NSG female mice were infused i.v. with 10x106 CAR-T cells and control NT-T cells. Mice were monitored for a total period of 250 days, by recurrent bleed. Simultaneously, another cohort of mice was infused with patient-derived BM cells (5x106) and monitored for the same time. Mice infused with Bcp-ALL BM cells developed leukemia-phenotype,with 82% of cells expressing hCD45dim and hCD19. By contrast, mice receiving patient-derived CAR-T cells showed a lowpercentage of CD45+ cells (0.1±0.01%), all CD3+. Despite the long period of observation, we did not detect any expansion of hCD19+ cells in this animal cohort.
Taken together these data suggest that the use of a retroviral platform, associated with the presence of iC9 suicide gene, contributes to the genesis of a highly functional and safe CAR-T product, even when the production starts from a biological material characterized by high contamination of leukemic blasts.
Locatelli:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Miltenyi: Honoraria; bluebird bio: Consultancy; Bellicum: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal